Seiji Torii 1, Chisato Kubota 1, Naoya Saito 1, Ayumi Kawano 1, Ni Hou 1, Masaki Kobayashi 2, Ryoko Torii 1, Masahiro Hosaka 3, Tadahiro Kitamura 2, Toshiyuki Takeuchi 1, and Hiroshi Gomi 4 (1: Secret Biol, IMCR, Gunma Univ.; 2: Metab Signal, IMCR, Gunma Univ.; 3: Akita Prefectural Univ.; 4: College of Bioresource Sciences, Nihon Univ.)
About
Glucose is a principal regulator of pancreatic β-cell survival and growth as well as insulin secretion. In recent years there has been increasing interest in the hypothesis that insulin secreted in response to elevated glucose exerts autocrine/paracrine effects through its own receptors (insulin receptor: IR). Despite the growing evidence for the significance of the IR/IRS2-mediated signaling pathways in β-cells, whether secreted insulin acts in an autocrine fashion remains controversial. In this paper we provide evidence suggesting that phogrin, a unique inactive protein tyrosine phosphatase, specifically regulates glucose-stimulated autocrine insulin signaling in pancreatic β-cells. Our data support the hypothesis that transient localization of phogrin onto the plasma membrane following glucose-stimulated insulin secretion enables autocrine insulin signaling through IR binding and regulation of PTP1B in specific pancreatic β-cells.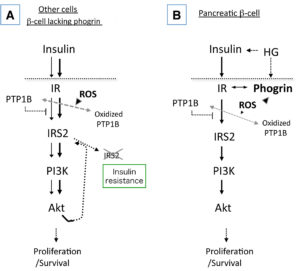
Paper information
The pseudophosphatase phogrin enables glucose-stimulated insulin signaling in pancreatic β-cells.
Seiji Torii, Chisato Kubota, Naoya Saito, Ayumi Kawano, Ni Hou, Masaki Kobayashi, Ryoko Torii, Masahiro Hosaka, Tadahiro Kitamura, Toshiyuki Takeuchi, and Hiroshi Gomi
J Biol Chem 293, 5920-5933, 2018 doi: 10.1074/jbc.RA117.000301
Online URL
https://www.ncbi.nlm.nih.gov/pubmed/29483197