Nakazawa S (Gunma Univ.), Oikawa D (Gunma Univ., Osaka City Univ.), Ishii R (Univ. of Tokyo), Ayaki T (Wakayama Med. Univ., Kyoto Univ.), Takahashi H (Ehime Univ.), Takeda H (Ehime Univ.), Ishitani R (Univ. of Tokyo), Kamei K (Gunma Univ.), Takeyoshi I (Gunma Univ.), Kawakami H (Hiroshima Univ.), Iwai K (Kyoto Univ.), Hatada I (Gunma Univ.), Sawasaki T (Ehime Univ.), Ito H (Wakayama Med. Univ.), Nureki O (Univ. of Tokyo), and Tokunaga F. (Gunma Univ., Osaka City Univ.)
About
Optineurin (OPTN) mutations cause neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS) and glaucoma. Although the ALS-associated E478G mutation in the UBAN domain of OPTN reportedly abolishes its NF-κB-suppressive activity, the precise molecular basis in ALS pathogenesis still remains unclear. Here we report that the OPTN-UBAN domain is crucial for NF-κB suppression. Our crystal structure analysis reveals that OPTN-UBAN binds linear ubiquitin with homology to NEMO. TNF-a-mediated NF-κB activation is enhanced in OPTN-knockout cells, through increased ubiquitination and association of TNF receptor complex I components. Furthermore, OPTN binds caspase 8, and OPTN-deficiency accelerates TNF-a-induced apoptosis by enhancing complex II formation. Immunohistochemical analyses of motor neurons from OPTN-associated ALS patients reveal that linear ubiquitin and activated NF-κB are partially colocalized with cytoplasmic inclusions, and that activation of caspases is elevated. Taken together, OPTN regulates both NF-κB activation and apoptosis via linear ubiquitin-binding, and the loss of this ability may lead to ALS.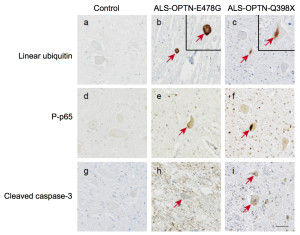
Paper information
Linear ubiquitination is involved in the pathogenesis of optineurin-associated amyotrophic lateral sclerosis. Nakazawa S, Oikawa D, Ishi R, Ayaki T, Takahashi H, Takeda H, Ishitani R, Kamei K, Takeyoshi I, Kawakami H, Iwai K, Hatada I, Sawasaki T, Ito H, Nureki O, and Tokunaga F. Nat. Commun. 7, 12547, 2016. doi: 10.1038/ncomms12547. PMID: 27552911
Online URL
https://www.ncbi.nlm.nih.gov/pubmed/27552911
Lab HP
http://www.med.osaka-cu.ac.jp/departments/bunshi-pathobiochemistry.shtml