Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Takeuchi T (IMCR, Gunma Univ.) Sasaki M, Suzuki T, Mori M (Grad Sch of Sci and Tech, Gunma Univ.) Yoshimoto Y (Gunma Univ Grad Sch of Med) Yamada K (Grad Sch of Sci and Tech, Gunma Univ.)
About
Pharmacological challenges to oncogenic Ras-expressing cancer cells have shown a novel type of cell death, ferroptosis, which requires intracellular iron. Here we assessed ferroptosis following treatment of human fibrosarcoma HT1080 cells with several inhibitors of lysosomal activity and found that they prevented cell death induced by the ferroptosis-inducing compounds (FINs). Fluorescent analyses with a reactive oxygen species (ROS) sensor revealed constitutive generation of ROS in lysosomes, and treatment with lysosome inhibitors decreased both lysosomal ROS and a ferroptotic cell death-associated ROS burst. These inhibitors partially prevented intracellular iron provision by attenuating intracellular transport of transferrin or autophagic degradation of ferritin. Furthermore, analyses with a fluorescent sensor that detects oxidative changes in cell membranes revealed that formation of lipid ROS in perinuclear compartments likely represented an early event in ferroptosis. These results suggest that lysosomal activity is involved in lipid ROS-mediated ferroptotic cell death through regulation of cellular iron equilibria and ROS generation.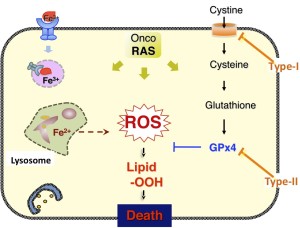
Paper information
An essential role for functional lysosomes in ferroptosis of cancer cells.
Torii S, Shintoku R, Kubota C, Yaegashi M, Torii R, Sasaki M, Suzuki T, Mori M, Yoshimoto Y, Takeuchi T, Yamada K.
Biochem J 473: 769-777 2016
Online URL
http://www.ncbi.nlm.nih.gov/pubmed/26759376