Shinnosuke Masuda1,#, Tetsuro Komatsu1, #, Safiya Atia1, Tomohiro Suzuki1, Mayuko Hayashi1, Atsushi Toyoda2, Hiroshi Kimura3, and Takeshi Inagaki1* (1Laboratory of Epigenetics and Metabolism, Institute for Molecular and Cellular Regulation, Gunma University, 2Advanced Genomics Center, National Institute of Genetics, 3Cell Biology Center, Institute of Integrated Research, Institute of Science Tokyo)
About
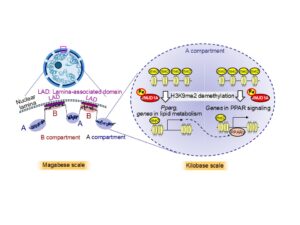
Iron serves as an essential cofactor for all JmjC-type histone demethylases, DNA demethylases, and RNA demethylases, playing a crucial role in broad epigenetic regulation. In this study, based on our recent findings that iron-dependent demethylation by epigenetic enzymes is indispensable for transcriptional regulation during adipocyte differentiation, we focused on JMJD1A, a histone demethylase targeting H3K9. Using an adipocyte differentiation model, we identified genes regulated by JMJD1A through iron-dependent demethylation of H3K9me2. Our results demonstrated that this iron-dependent epigenetic regulation was predominantly observed in the structurally relaxed A compartments, where JMJD1A reduced H3K9me2 levels on a kilobase scale around transcription start sites (TSSs), thereby targeting genes involved in PPAR signaling and lipid metabolism in an iron-dependent manner.
Paper information
Masuda S, Komatsu T, Atia S, Suzuki T, Hayashi M, Toyoda A, Kimura H, Inagaki T. Iron-dependent JMJD1A-mediated Demethylation of H3K9me2 Regulates Gene Expression During Adipogenesis in a Spatial Genome Organization-dependent Manner. Genes to Cells 2025 (doi.org/10.1111/gtc.70023)
Online URL
https://onlinelibrary.wiley.com/doi/10.1111/gtc.70023