Takahiro Tsuno 1,2, Jinghe Li 1, Kuniyuki Nishiyama 1, Yuka Imamura Kawasawa 3, Ryota Inoue 1, Esther Ong Yajima 1, Akira Nishiyama 4, Shigeharu G. Yabe 5, Tatsuya Kin 6, Hitoshi Okochi 5, Tomohiko Tamura 4,7, A. M. James Shapiro 6, Seiichi Oyadomari 8, Tadahiro Kitamura 9, Yasuo Terauchi 2, and Jun Shirakawa 1,2,* (1. Laboratory of Diabetes and Metabolic Disorders, Institute for Molecular and Cellular Regulation (IMCR), Gunma University, Japan; 2. Department of Endocrinology and Metabolism, Graduate School of Medicine, Yokohama City University, Japan; 3. Penn State University College of Medicine, Hershey, PA, USA; 4. Department of Immunology, Graduate School of Medicine, Yokohama City University, Japan; 5. Department of Regenerative Medicine, National Center for Global Health and Medicine (NCGM), Japan; 6. Clinical Islet Laboratory and Clinical Islet Transplant Program, University of Alberta, Canada; 7. Advanced Medical Research Center, Yokohama City University, Japan; 8. Division of Molecular Biology, Institute of Advanced Medical Sciences, Tokushima University, Japan; 9. Metabolic Signal Research Center, Institute for Molecular and Cellular Regulation (IMCR), Gunma University, Japan; *Corresponding Author)
About
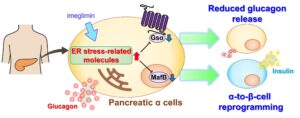
Dysregulated α-cell function contributes to the development of diabetes. In this study, we found that treatment with imeglimin, an antidiabetic drug, prevents glucagon release and induces a loss of α-cell identity through direct action on α cells. Mechanistically, imeglimin reduces Gsα expression to inhibit the EPAC2-mediated secretion of glucagon induced by low glucose, GIP, or adrenaline in an insulin-independent manner. Imeglimin also attenuates α-cell Ca2+ oscillations. MafB expression is downregulated by imeglimin to induce α-cell dedifferentiation. In addition, imeglimin upregulates CHOP expression, which partly contributes to the reduction in Gsα and MafB expression to reduce glucagon secretion and induce α-cell reprogramming without altering protein translation. These pleiotropic effects of imeglimin on glucagon secretion and α-cell identity can be recapitulated in mouse models of diabetes in vivo. These data suggest that the imeglimin-mediated regulation of α-cell plasticity, particularly via glucagon suppression, may contribute to glucose homeostasis.
Paper information
Imeglimin suppresses glucagon secretion and induces a loss of α-cell identity
Takahiro Tsuno 1,2, Jinghe Li 1, Kuniyuki Nishiyama 1, Yuka Imamura Kawasawa 3, Ryota Inoue 1, Esther Ong Yajima 1, Akira Nishiyama 4, Shigeharu G. Yabe 5, Tatsuya Kin 6, Hitoshi Okochi 5, Tomohiko Tamura 4,7, A. M. James Shapiro 6, Seiichi Oyadomari 8, Tadahiro Kitamura 9, Yasuo Terauchi 2, and Jun Shirakawa 1,2,*
(1. Laboratory of Diabetes and Metabolic Disorders, Institute for Molecular and Cellular Regulation (IMCR), Gunma University, Japan; 2. Department of Endocrinology and Metabolism, Graduate School of Medicine, Yokohama City University, Japan; 3. Penn State University College of Medicine, Hershey, PA, USA; 4. Department of Immunology, Graduate School of Medicine, Yokohama City University, Japan; 5. Department of Regenerative Medicine, National Center for Global Health and Medicine (NCGM), Japan; 6. Clinical Islet Laboratory and Clinical Islet Transplant Program, University of Alberta, Canada; 7. Advanced Medical Research Center, Yokohama City University, Japan; 8. Division of Molecular Biology, Institute of Advanced Medical Sciences, Tokushima University, Japan; 9. Metabolic Signal Research Center, Institute for Molecular and Cellular Regulation (IMCR), Gunma University, Japan; *Corresponding Author)
Online URL
https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00327-1