Takuro Horii1*, Sumiyo Morita1, Shinjiro Hino2, Yuko Hino2, Hiroto S Fukushima3, Ryosuke Kobayashi1, Mika Kimura1, Mitsuyoshi Nakao2, Yoichi Mizukami4, Azusa Inoue3,5 and Izuho Hatada1,6* (1. Laboratory of Genome Science, Biosignal Genome Resource Center, Institute for Molecular and Cellular Regulation, Gunma University; 2. Department of Medical Cell Biology, Institute of Molecular Embryology and Genetics, Kumamoto University; 3. Laboratory for Epigenome Inheritance, RIKEN Center for Integrative Medical Sciences; 4. Institute of Gene Research, Yamaguchi University Science Research Center; 5. Tokyo Metropolitan University, 6. Viral Vector Core, Gunma University Initiative for Advanced Research; *Corresponding Author)
About
Traditionally, it has been believed that parental traits are inherited by offspring through the nucleotide sequence of genomic DNA. However, recent studies suggest that stress applied to parents can affect the epigenome of gametes, influencing the health and constitution of their children and grandchildren. It has remained unclear whether these epigenomic changes are directly inherited or whether other factors are involved.
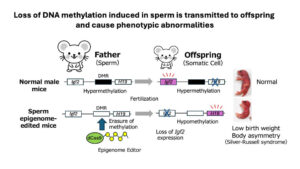
In this study, we developed a “sperm-specific epigenome editing system” that erases DNA methylation and examined it in mice. The target region, H19-DMR, is known to exhibit epigenomic abnormalities in patients with Silver-Russell syndrome and is normally hypermethylated in sperm. Using this system, we successfully reduced methylation levels in this region. As a result, part of the reduced methylation state in sperm was inherited by offspring, which exhibited characteristics such as low birth weight and body asymmetry, similar to Silver-Russell syndrome. However, not all abnormalities were inherited; some were restored after fertilization by a safety mechanism involving histone modification H3K9me3.
This study is the first in the world to directly demonstrate that changes other than DNA sequence can be transmitted to offspring and contribute to disease onset, offering new insights into disease mechanisms and potential applications in prevention and treatment development.
Paper information
Horii T, Morita S, Hino S, Hino Y, Fukushima H, Kobayashi R, Kimura M, Nakao M, Mizukami Y, Inoue A, Hatada I. Germline epigenome editing identifies H3K9me3 as a mediator of intergenerational DNA methylation recovery in mice.
Nat Commun. 2025 Dec.
Online URL
https://www.doi.org/10.1038/s41467-025-67488-9