Takuya Norizuki1,#, Yasuharu Kushida2,#, Takayuki Sekimoto1, Taeko Sasaki1, Koji Yamano3,4, Noriyuki Matsuda3, Ryohei Sasaki5, Nobuo N. Noda5,6,*, Ken Sato2,*, and Miyuki Sato1,* (1. Laboratory of Molecular Membrane Biology, Institute for Molecular and Cellular Regulation, Gunma University; 2. Laboratory of Molecular Traffic, Institute for Molecular and Cellular Regulation, Gunma University; 3. Department of Biomolecular Pathogenesis, Medical Research Laboratory, Institute of Integrated Research, Institute of Science Tokyo; 4. Intracellular Quality Control Project, Tokyo Metropolitan Institute of Medical Science; 5. Institute for Genetic Medicine, Hokkaido University; 6. Institute of Microbial Chemistry; #Co-first author; *Corresponding author)
About
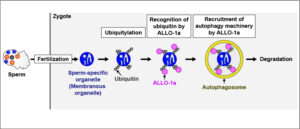
After fertilization, intracellular components of sperm enter the egg cytoplasm. We previously showed that some of these components are degraded by autophagy, and that ALLO-1 is responsible for their recognition in the nematode Caenorhabditis elegans. However, the underlying mechanism has remained unclear.
In this study, we show that ALLO-1a interacts with ubiquitin based on in silico structural prediction and biochemical analyses, and that this interaction is indispensable for the degradation of sperm-specific organelles called membranous organelles in C. elegans. Our finding highlights a ubiquitin-mediated mechanism that underlies the recognition of sperm-specific organelles after C. elegans fertilization.
Paper information
Norizuki, T., Kushida, Y., Sekimoto, T., Sasaki, T., Yamano, K., Matsuda, N., Sasaki, R., Noda, N.N., Sato, K., and Sato, M. ALLO-1a is a ubiquitin-binding adaptor for allophagy in Caenorhabditis elegans. J. Cell Sci.