Mizuno K, Fujita T, Gomi H, Izumi T (Laboratory of Molecular Endocrinology and Metabolism, IMCR、Gunma University)
About
After stimulation with secretagogues, insulin granules are fused with plasma membrane to release insulin from pancreatic β cells. Here we directly observed dynamics of a docking factor, granuphilin, and insulin granule exocytosis in living cells under total internal reflection fluorescence microscopy (TIRFM). We found that, although granuphilin has a fusion-inhibitory activity to suppress spontaneous fusion of docked granules, it functionally permits their fusion after stimulation. We also measured nano-architecture of docking machinery by super-resolution fluorescence microscopy, dSTORM, and found that granuphilin forms a 180-nm cluster on each 350-nm granule on the plasma membrane. Our findings demonstrate that granuphilin is an essential component of the docking machinery and that granuphilin-mediated docking is neither a prerequisite for fusion nor an off-pathway leading to a dead end.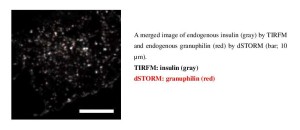
Paper information
Mizuno K, Fujita T, Gomi H, and Izumi T (2016). Granuphilin exclusively mediates functional granule docking to the plasma membrane. Sci. Rep., 6, 23909. doi: 10.1038/srep23909. PMID: 27032672
Online URL
http://www.nature.com/articles/srep23909